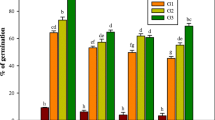
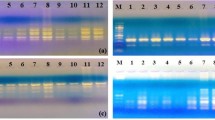
Abstract
Vigna radiata (L.) Wilczek, popularly known as “mung bean,” is an important protein supplement in vegetarian diets in many countries of Asia. It has a short life cycle (55–70 days) and fits well into many cropping systems, including rice and sugarcane, under rain-fed and irrigated conditions. The present review deals with the data available on plant regeneration of this species. Both shoot-tip multiplication and somatic embryogenesis have been compared on the basis of retrospective as well as recent reports. Molecular markers, especially random amplified polymorphic DNA (RAPD), have been used to compare results obtained from in vitro and in vivo studies on various species ofVigna. Isozyme markers such as esterase and superoxide dismutase, which are expressed during in vitro regeneration and in vivo development ofVigna species, are also included in this article.
Similar content being viewed by others
Literature Cited
Ahuja, B. S., T. A. Sharma Ushakiran &Sundershan. 1980. Activities of Superoxide dismutase & peroxidase enzymes during early phase of germination in mung bean (Phaseolus aureus). Indian J. Biochem. Biophys. 17: 77–79.
Bajaj, Y. P. S. &M. S. Dhanju. 1979. Regeneration of plants from apical meristem tips of some legumes. Curr. Sci. 48: 906–907.
— &H. Singh. 1980.In vitro induction of androgenesis in mung bean (Phaseolus aureus L.). Indian J. Exp. Biol. 18: 1316–1318.
Baum, J. A. &J. G. Scandalios. 1981. Isolation and characterization of the cytosolic and mitochondrial Superoxide dismutases of maize. Arch. Biochem. Biophys. 206: 249–264.
—,J. M. Chandlee &J. G. Scandalios. 1983. Purification and partial characterization of a genetically-defined Superoxide dismutase (SOD-1) associated with maize chloroplasts. Plant Physiol. 73: 31–35.
Beauchamp, C. O. &I. Fridovich. 1973. Isozymes of Superoxide dismutase from wheat germ. Biochem. Biophys. Acta 317: 50–64.
Betal, S. 2003. In vitro plant regeneration and molecular biological studies ofVigna radiata (L.) Wilczek. Ph.D. diss., Calcutta University.
— &S. Sen Raychaudhuri. 1999. Micropropagation of the endangered aromatic varieties ofVigna radiata (L.) Wilczek in West Bengal, India. In Vitro Cell Dev. Biol.-Plant 35: 76–78.
——. 2001. Shoot tip multiplication in aromatic and non-aromatic varieties ofVigna radiata (L.) Wilczek. Plant Tissue Cult. 11(2): 187–194.
Bora, K. K., R. Ganesh & S. R. Mathus. 1999. Effect of triazole on the activities of enzymes of activated oxygen metabolism and lipid peroxidation in senescing mungbean leaves. Abstr. 5773, XVI Intl. Bot. Congr., Saint Louis, MO.
Boutin, S. R., N. D. Young, T. C. Olson, Z. H. Yu, R. C. Shoemaker &C. E. Vallejos. 1995. Genome conservation among three legume genera detected with DNA markers. Genome 38: 928–937.
Chakraborty, S. 1987. Cytological and biochemical aspects of cultured tissues during growth and regeneration and effect of mutagens on cabbage underin vitro. Ph.D. diss., Calcutta Univ.
Chandra, M. &A. Pal. 1995. Differential response of the two cotyledons ofVigna radiata in vitro. Plant Cell Rep. 15: 248–253.
Chandra, S. 1988. Problems and prospects of mungbean improvement in India. Pp. 588–595in Mungbean: Proceedings of the Second International Symposium, Bangkok, Thailand, 1987.
Cheng, T. Y., H. Saka &T. H. Voqui-Dinh. 1980. Plant regeneration from soybean cotyledonary node segments in culture. Plant Sc. Lett. 19: 91–99.
Cubadda, R. &E. Quattrucci. 1974. Separation by gel electrofocussing and characterization of wheat esterses. J. Sci. Food Agric. 25: 417–422.
Eapen, S. 1988. Callus induction from mesophyll and hypocotyl protoplasts of mungbean (Vigna radiata (L.)). Ann. Bot. 62: 441–443.
Fatokun, C. A., D. I. Danesh, D. Menancio-Hautea &N. D. Young. 1992. A linkage map of cowpea [Vigna unguiculata (L.) Walp] based on DNA markers (2N = 22). Pp. 6.256–6.258in S. J. O’Brien (ed.), Genome maps. Ed. 6. Cold Spring Harbor Laboratory Press, Plainview, NY.
Fernandez, G. C. J. & S. Shanmugasundaram. 1988. The AVRDC Mungbean Improvement Program: The past present and future. Pp. 58–70in Mungbean: Proceedings of the Second International Symposium, Bangkok, Thailand, 1987.
Franklin, C. I., T. N. Trieu, R. A. Gonzales &R. A. Dixon. 1991. Plant regeneration from seedling expiants of green bean (Phaseolus vulgaris L.) via organogenesis. Plant Cell Tissue Org. Cult. 24: 199–206.
Fridovich, I. 1975. Superoxide dismutases. Annual Rev. Biochem. 44: 147–159.
—. 1978. The biology of oxygen radicals. Science 201: 875–880.
Gamborg, O. L., R. A. Miller &K. Ojima. 1968. Nutrient requirements of suspension cultures of soybean root cells. Exp. Cell Res. 50: 151–158.
Girija, S. &A. Ganapathi. 1996. Somatic embryogenesis in mungbean (Vigna radiata L. Wilczek). In Vitro 32(2): 86A.
Gulati, A. &P. K. Jaiwal. 1990. Culture conditions affecting plant regeneration from cotyledon of mungbean (Vigna radiata L. Wilczek). Plant Cell Tissue Org. Cult. 23: 1–7.
——. 1992.In vitro induction of multiple shoots and plant regeneration from shoot tips of mungbean (Vigna radiata). Plant Cell Tissue Org. Cult. 29: 199–205.
——. 1994. Plant regeneration from cotyledonary node expiants of mungbean (Vigna radiata (L.) Wilczek). Plant Cell Rep. 13: 523–527.
Jackson, C., J. Dench, A. L. Moore, B. Halliwell, C. H. Foyer &D. O. Hall. 1978. Subcellular localization and identification of Superoxide dismutase in the leaves of higher plants. Eur. J. Biochem. 91: 339–344.
Jackson, J. A. &S. L. A. Hobbs. 1990. Rapid multiple shoot production from cotyledonary node explants pf pea (Pisum sativum L.). In Vitro Cell Dev. Biol.-Plant 26: 835–838.
Jain, H. K. &K. L. Mehra. 1980. Evolution, adaptation, relationships and uses of the species ofVigna cultivated in Asia. Pp. 459–468in R. J. Summerfield & A. H. Bunting (eds.), Advances in legume science. Royal Botanic Gardens, Kew, England.
Kumar, V. &D. R. Sharma. 1989. Isolation and characterization of sodium chloride resistant callus culture ofVigna radiata (L.) Wilczek var. radiata. J. Exp. Bot. 40(210): 143–147.
Lambrides, C. J., R. J. Lawn, I. D. Godwin, J. Manners &B. C. Imrie. 2000. Two genetic linkage maps of mungbean using RFLP and RAPD markers. Australian J. Agric. Res. 51: 415–25.
Lanham, P. G. 1996. Estimation of heterozygosity inRibes nigrum L. using RAPD markers. Genetica 98: 193–197.
Ling, J. R., R. Sanve &N. Gawel. 1997. Identification of poinsettia cultivars using RAPD markers. Hort. Sci. 32(1): 122–124.
Machado, M. A., H. D. Coletta, M. L. P. N. Targon &J. Pompeu. 1996. Genetic relationship of Mediterranean mandarins (Citrus deliciosa Terore) using RAPD markers. Euphytica 92: 321–326.
Malik, C. P. &M. B. Singh. 1994. Plant enzymology and histo-enzymology—A text manual. Kalyani Publishers, Ludhiana, India.
Martinez, C. A., M. E. Loureiro, M. A. Oliva &M. Maestri. 2001. Differential responses of Superoxide dismutase in freezing resistantSolanum curtilobum and freezing sensitiveSolanum tuberosum subjected to oxidative and water stress. Plant Sci. 160: 505–515.
Mathews, H. 1987. Morphogenetic responses forin vitro cultured seedling expiants of mungbean (Vigna radiata L. Wilczek). Plant Cell Tissue Org. Cult. 11: 163–168.
McClean, P. &K. F. Grafton. 1989. Regeneration of dry bean (Phaselus vulgaris L.) via organogenesis. Plant Sci. 60: 117–122.
McCord, J. M. &I. Fridovich. 1969. Superoxide dismutase: An enzymic function for erythrocuprein (Hemocuprein). J. Biol. Chem. 244: 6049–6055.
—,B. B. Keele &I. Fridovich. 1971. An enzyme based theory of obligate anaerobiosis: The physiological function of Superoxide dismutase. Proc. Natl. Acad. U.S.A. 68: 1024–1027.
Menancio-Hautea, D., L. Kumar, D. Danesh &N. D. Young. 1992. A genome map for mungbean [Vigna radiata (L.) Wilczek] based on DNA genetic markers (2N = 2X = 22). Pp. 6.259–6.261in S. J. O’Brien (ed.), Genome maps. Ed. 6. Cold Spring Harbor Laboratory Press, Plainview, NY.
—,C. A. Fatokun, L. Kumar, D. Danesh &N. D. Young. 1993. Comparative genome analysis of mungbean (Vigna radiata L. Wilczek) and cowpea (V. unguiculata L. Walpers) using RFLP mapping data. Theor. Appl. Genet. 86(7): 797–810.
Mendoza, A. B., K. Hattori, T. Nishimura &Y. Futsuhara. 1993. Histological and scanning electron microscopic observations on plant regeneration in mungbean cotyledon (Vigna radiata (L.) Wilczek) culturedin vitro. Plant Cell Tissue Org. Cult. 32: 137–43.
Mroginski, L. A. &K. K. Kartha. 1984. Tissue culture of legumes for crop improvement. Pp. 215–264in J. Janick (ed.), Plant breeding reviews. AVI Publishing Co., Westport, CT.
Mullis, K. B., S. Faloona, R. Saiki, G. Horn &H. Erlich. 1986. Specific enzymatic amplification of DNAin vitro: The polymerase chain reaction. Cold Spring Harbor Symp. Quant. Biol. 51: 263–273.
Murashige, T. &F. Skoog. 1962. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15: 473–497.
Pal, M., U. Ghosh, M. Chandra, A. Pal &B. B. Biswas. 1991. Transformation and regeneration of mungbean (Vigna radiata). Indian J. Biochem. Biophys. 28: 449–455.
Paroda, R. S. & T. A. Thomas. 1988. Genetic resources of mungbean (Vigna radiata (L.) Wilczek) in India. Pp. 19–28in Mungbean: Proceedings of the Second International Symposium, Bangkok, Thailand, 1987.
Pasquet, R. S. 1999. Genetic relationships among subspecies ofVigna unguiculata (L.) Walp. based on allozyme variation. Theor. Appl. Genet. 98: 1104–1119.
Patel, M. B., R. Bhardwaj &A. Joshi. 1991. Organogenesis inVigna radiata (L.) Wilczek. Indian J. Exp. Biol. 29: 619–622.
Perl-Treves, R. &E. Galun. 1991. The tomato Cu, Zn Superoxide dismutase genes are developmentally regulated and respond to light stress. Plant Mol. Biol. 17: 45–60.
Pramanik, S., S. Chakraborty &S. Sen Raychaudhuri. 1994. Nuclear DNA content and chromosomal variation in relation to callus growth duringin vitro regeneration inPlantago ovata. Cytobios 80: 101–108.
—,S. Sen Raychaudhuri &S. Chakraborty. 1996. Changes in isozymes duringin vitro morphogenesis inPlantago ovata Forsk. Plant Cell Tissue Org. Cult. 44: 123–127.
Raghunathachari, P., V. K. Khanna, U. S. Singh &N. K. Singh. 2000. RAPD analysis of genetic variability in Indian scented rice germplasm (Oryza sativa L.). Curr. Sci. 79 (7): 994–998.
Reddy, C. D. &B. Venkaiah. 1984. Studies on Superoxide dismutase from mungbean (Vigna radiata) seedlings. J. Plant Physiol. 116: 279–284.
Sandhu, T. S., K. Singh &B. Singh. 1988. Mungbean germplasm resources: Evaluation and utilization. Pp. 29–34in Mungbean: Proceedings of the Second International Symposium, Bangkok, Thailand, 1987.
Sawada, Y., T. Ohyama &I. Yamazaki. 1972. Preparation and physicochemical properties of green pea Superoxide dismutase. Biochem. Biophys. Acta 268: 305–312.
Selva, E., B. Stouffs &M. Briquet. 1989. In vitro propagation ofVicia faba L. by micro-cutting and multiple shoot induction. Plant Cell Tissue Org. Cult. 18(2): 167–169.
Selvi, D. T., N. M. Ramaswamy, S. Sukumar &S. R. Sree Rangaswamy. 1995.In vitro regeneration and protoplast culture studies in mungbean (Vigna radiata (L.) Wilczek). In Vitro 31(2): 62A.
Singh, D. P. 1988. Current status of mungbean yellow mosaic virus resistance breeding. Pp. 282–289in Mungbean: Proceedings of the Second International Symposium, Bangkok, Thailand, 1987.
Singh, G., S. Kapoor & K. Singh. 1988. Multiple disease resistance in mungbean with special emphasis on mungbean yellow mosaic virus. Pp. 290–296in Mungbean: Proceedings of the Second International Symposium, Bangkok, Thailand, 1987.
Sonnante, G., A. R. Piergiovanni, N. Q. Ng &P. Perrino. 1996. Relationships ofVigna unguiculata (L.) Walp.,V. vexillata (L.) A. Rich and species of sectionVigna based on isozyme variation. Genetic Resources & Crop Evolution 43: 157–165.
—,A. Spinosa, A. Marangi &D. Pignone. 1997. Isozyme and RAPD analysis of the genetic diversity within and betweenVigna luteola andVigna marina. Ann. Bot. 80: 741–746.
Staub, J., J. Bacher &K. Poetter. 1996. Sources of potential errors in the application of random amplified polymorphic DNAs in cucumber. Hort. Sci. 31(2): 262–266.
VCGC (Vigna Crop Germplasm Committee). 1996.Vigna germplasm current status and future needs: A report prepared by theVigna Crop Germplasm Committee, March 1996.
Wilde, L. G. &M. Yu. 1998. Effect of fluoride on Superoxide dismutase (SOD) activity in germinating mungbean seedlings. Fluoride 31(2): 81–88.
Williams, J. G. K., A. R. Kubelik, K. J. Livak, J. A. Rafalski &S. V. Tingey. 1990. DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res. 18: 6531–6535.
Wright, M. S., S. M. Koehler, M. G. Carner, M. H. Williams, M. A. Hinchee, S. M. Colburn, G. C. Devis &P. E. Pierson. 1986. Plant regeneration from tissue cultures of soybean by organogenesis. Pp. 3: 111–119 in I. K. Vasil (ed.), Cell culture and somatic cell genetics of plants: Plant regeneration and genetic variability. Academic Press, New York.
Xu, H. &A. T. Bakalinsky. 1996. Identification of grape (Vitis) rootstock using sequence characterized amplified region DNA markers. Hort. Sci. 31(2): 267–268.
Young, N. D., D. Danesh, D. Menancio-Hautea &L. Kumar. 1993. Mapping oligogenic resistance to powdery mildew in mungbean with RFLPs. Theor. Appl. Genet. 87: 243–249.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Betal, S., Raychaudhuri, S.S. Isozyme and RAPD markers in relation to in vitro morphogenesis and in vivo development ofVigna radiata (L.) Wilczek. Bot. Rev 69, 441–456 (2003). https://doi.org/10.1663/0006-8101(2004)069[0441:IARMIR]2.0.CO;2
Issue Date:
DOI: https://doi.org/10.1663/0006-8101(2004)069[0441:IARMIR]2.0.CO;2